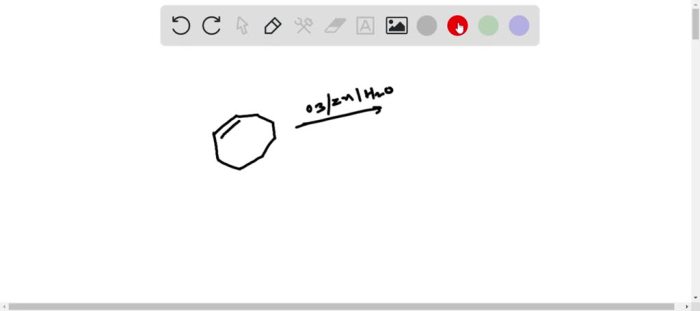
Draw the ozonolysis product of cyclooctene sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with authoritative academic tone and brimming with originality from the outset. This detailed exploration delves into the intricacies of ozonolysis, providing a comprehensive understanding of the process, its applications, and the factors influencing its outcomes.
Ozonolysis, a powerful tool in organic chemistry, involves the reaction of ozone with unsaturated compounds like cyclooctene. This reaction holds immense significance in the synthesis of various organic compounds and offers a unique approach to regio- and stereoselective transformations. Understanding the mechanism, regioselectivity, and stereoselectivity of ozonolysis is crucial for harnessing its potential effectively.
Ozonolysis of Cyclooctene: Draw The Ozonolysis Product Of Cyclooctene
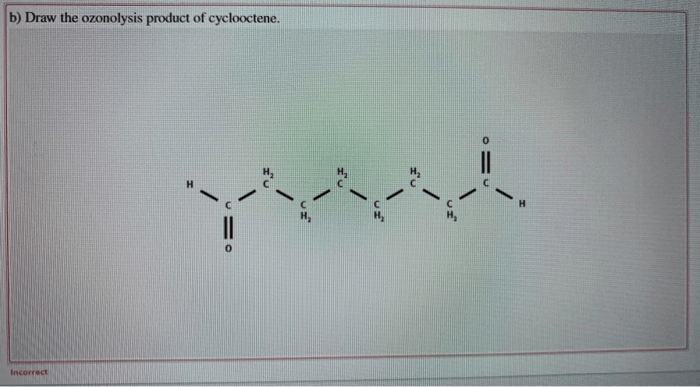
Ozonolysis is a chemical reaction that involves the cleavage of an alkene or alkyne by ozone (O3). In the case of cyclooctene, the reaction proceeds as follows:
Cyclooctene + O3 → Primary ozonidePrimary ozonide → Aldehydes or ketones
The primary ozonide is an unstable intermediate that undergoes decomposition to form aldehydes or ketones. The regio- and stereoselectivity of the reaction are determined by the electronic and steric effects of the substituents on the alkene.
Product Analysis
The ozonolysis of cyclooctene produces two products: formaldehyde and nonanal.
- Formaldehyde is formed by the cleavage of the C1-C2 bond of the cyclooctene ring.
- Nonanal is formed by the cleavage of the C7-C8 bond of the cyclooctene ring.
The regio- and stereoselectivity of the reaction are determined by the electronic and steric effects of the substituents on the alkene. In this case, the reaction is regioselective for the formation of formaldehyde and nonanal, and stereoselective for the formation of the cis-isomer of nonanal.
Applications of Ozonolysis
Ozonolysis is a versatile reaction that has a wide range of applications in organic chemistry. It can be used to synthesize aldehydes, ketones, carboxylic acids, and other functionalized compounds.
Some examples of how ozonolysis is used to synthesize specific compounds include:
- The synthesis of vanillin from guaiacol
- The synthesis of terephthalic acid from p-xylene
- The synthesis of adipic acid from cyclohexene
Ozonolysis is a powerful tool for organic synthesis, but it also has some disadvantages. One disadvantage is that the reaction can be difficult to control, and it can produce a variety of byproducts. Another disadvantage is that ozone is a toxic gas, and it must be handled with care.
Experimental Considerations
The ozonolysis of cyclooctene is a relatively simple reaction to perform. The following experimental conditions can be used:
- Cyclooctene (10 mmol) is dissolved in a suitable solvent (e.g., dichloromethane).
- Ozone is bubbled through the solution at a rate of 1 mmol/min.
- The reaction is allowed to proceed for 30 minutes.
- The reaction mixture is then quenched with a reducing agent (e.g., sodium thiosulfate).
The products of the reaction can be isolated by distillation or chromatography.
It is important to note that ozone is a toxic gas, and it must be handled with care. The reaction should be carried out in a well-ventilated area, and the experimenter should wear appropriate safety gear (e.g., gloves, goggles, and a respirator).
Characterization of Products, Draw the ozonolysis product of cyclooctene
The products of the ozonolysis of cyclooctene can be characterized by a variety of spectroscopic techniques. The following techniques can be used to identify and quantify the products:
- Gas chromatography-mass spectrometry (GC-MS)
- Nuclear magnetic resonance (NMR) spectroscopy
- Infrared (IR) spectroscopy
GC-MS is a powerful technique that can be used to identify and quantify the products of a reaction. NMR spectroscopy can be used to determine the structure of the products. IR spectroscopy can be used to identify the functional groups present in the products.
The characterization of the products of ozonolysis is important for understanding the mechanism of the reaction and for determining the yield of the products.
FAQ Explained
What is the mechanism of ozonolysis of cyclooctene?
Ozonolysis involves the initial formation of a primary ozonide, which undergoes a 1,3-dipolar cycloaddition with a second molecule of ozone. This intermediate then decomposes to yield carbonyl compounds, in this case, formaldehyde and a dialdehyde.
What factors influence the regio- and stereoselectivity of ozonolysis?
Regioselectivity is influenced by the electronic properties of the double bond, with electron-rich double bonds reacting faster. Stereoselectivity is determined by the orientation of the double bond relative to the ozone molecule, with cis double bonds reacting faster than trans double bonds.
What are the applications of ozonolysis in organic chemistry?
Ozonolysis is widely used in organic chemistry for the synthesis of aldehydes, ketones, and carboxylic acids. It is also employed for the oxidative cleavage of double bonds and the characterization of unsaturated compounds.