Which molecule listed below is a nonpolar molecule – In the realm of chemistry, understanding the polarity of molecules is crucial for comprehending their behavior and interactions. This article delves into the fascinating world of nonpolar molecules, exploring their unique characteristics, identification methods, and practical applications.
Nonpolar molecules, devoid of a permanent dipole moment, play a vital role in various scientific and industrial domains. Their distinct properties render them indispensable for a wide range of applications, from solvents and lubricants to insulators and beyond.
Nonpolar Molecules
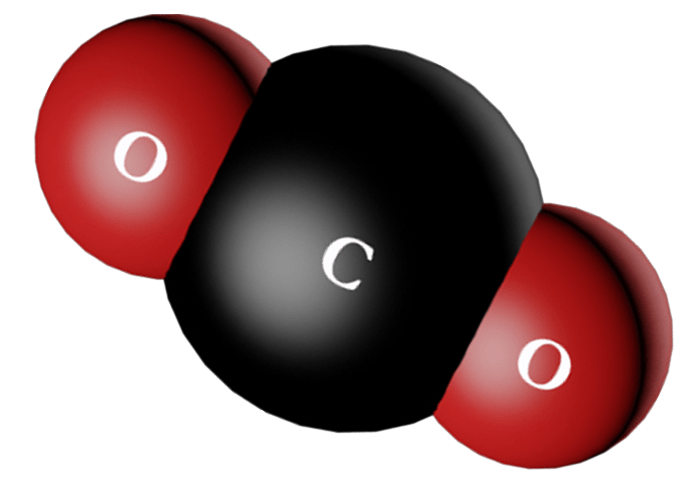
Nonpolar molecules are molecules that do not have a permanent dipole moment. This means that the electrons in the molecule are evenly distributed, and there is no net positive or negative charge on the molecule.
Identifying Nonpolar Molecules, Which molecule listed below is a nonpolar molecule
There are a few properties that distinguish nonpolar molecules from polar molecules. First, nonpolar molecules are typically composed of nonpolar atoms. Nonpolar atoms are atoms that have the same electronegativity, which is a measure of how strongly an atom attracts electrons.
Second, nonpolar molecules typically have a symmetrical shape. This means that the electrons in the molecule are evenly distributed around the molecule.
Examples of Nonpolar Molecules
- Hydrogen (H 2)
- Oxygen (O 2)
- Nitrogen (N 2)
- Carbon dioxide (CO 2)
- Methane (CH 4)
- Ethane (C 2H 6)
- Propane (C 3H 8)
Applications of Nonpolar Molecules
Nonpolar molecules have a wide range of applications. They are used as solvents, lubricants, and insulators. Nonpolar solvents are used to dissolve nonpolar substances. Nonpolar lubricants are used to reduce friction between moving parts. Nonpolar insulators are used to prevent the flow of electricity.
Comparison with Polar Molecules
Nonpolar molecules are different from polar molecules in several ways. First, nonpolar molecules do not have a permanent dipole moment, while polar molecules do. Second, nonpolar molecules are typically composed of nonpolar atoms, while polar molecules are typically composed of polar atoms.
Third, nonpolar molecules typically have a symmetrical shape, while polar molecules typically have an asymmetrical shape.
FAQ Insights: Which Molecule Listed Below Is A Nonpolar Molecule
What are the key characteristics of nonpolar molecules?
Nonpolar molecules exhibit a uniform distribution of electrons, resulting in no net dipole moment. They possess symmetrical structures and weak intermolecular forces.
How can electronegativity be used to determine molecular polarity?
Electronegativity measures the ability of atoms to attract electrons. Nonpolar molecules have atoms with similar electronegativities, leading to a balanced distribution of electrons.
Provide examples of common nonpolar molecules.
Nonpolar molecules include methane (CH4), carbon dioxide (CO2), and hexane (C6H14).