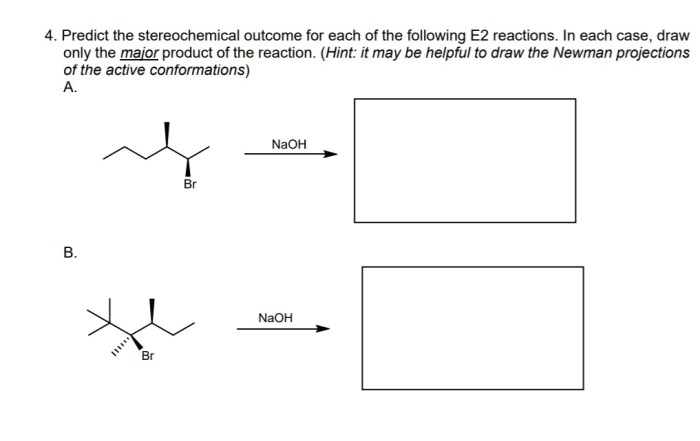
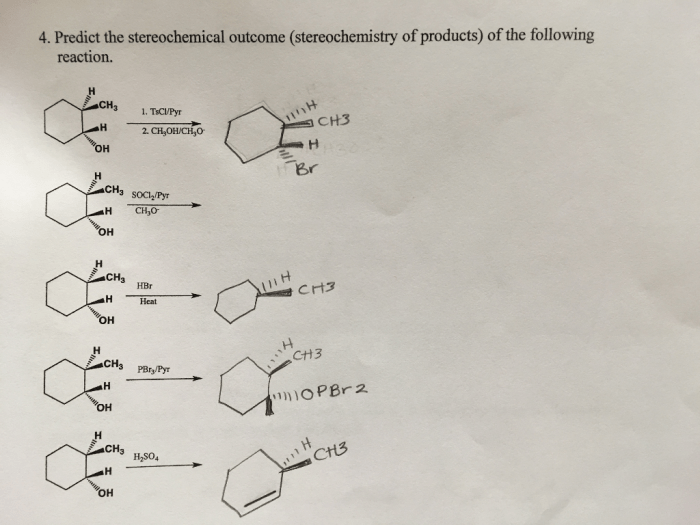
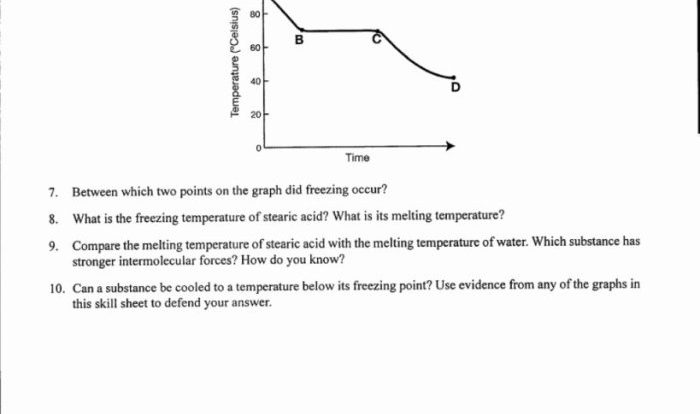
Predict the stereochemical outcome of the following reaction – Stereochemical outcomes play a crucial role in various scientific disciplines, and predicting them accurately is essential for understanding and controlling chemical reactions. This guide provides an in-depth exploration of stereochemical outcomes, their significance, and the methods used to predict them.
Stereochemical outcomes refer to the three-dimensional arrangement of atoms within a molecule. Different arrangements can result in different physical and chemical properties, making it critical to predict the stereochemical outcome of a reaction before it is carried out.
Overview: Predict The Stereochemical Outcome Of The Following Reaction
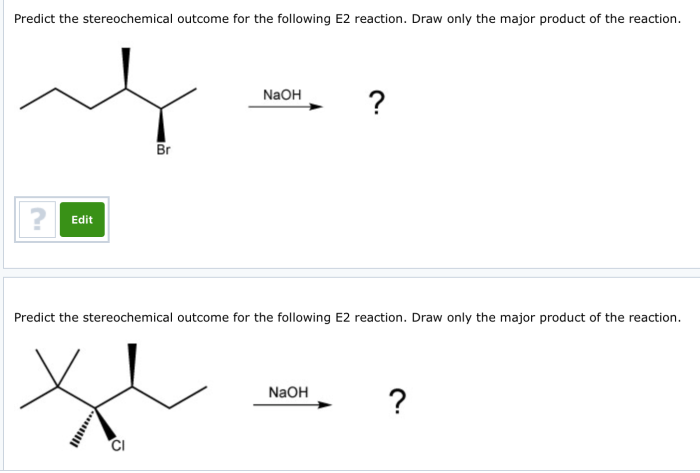
Stereochemical outcome refers to the spatial arrangement of atoms or groups in a molecule. It is crucial in various fields, including organic chemistry, biochemistry, and drug design. Understanding the stereochemical outcome of reactions allows chemists to predict the properties and reactivity of molecules.
There are different types of stereochemical outcomes that can occur in reactions. These include enantioselectivity, diastereoselectivity, and regioselectivity. Enantioselectivity refers to the formation of one enantiomer over the other in a reaction. Diastereoselectivity refers to the formation of one diastereomer over the others in a reaction.
Regioselectivity refers to the formation of one regioisomer over the others in a reaction.
Factors Influencing Stereochemical Outcome
Several factors can influence the stereochemical outcome of a reaction. These include:
- Substrate structure:The structure of the substrate can influence the stereochemical outcome of a reaction. For example, a substrate with a chiral center will react differently than a substrate without a chiral center.
- Reaction conditions:The reaction conditions, such as temperature, solvent, and catalyst, can also influence the stereochemical outcome of a reaction. For example, a reaction that is carried out at a low temperature is more likely to produce a stereoselective product than a reaction that is carried out at a high temperature.
- Catalyst:The catalyst used in a reaction can also influence the stereochemical outcome of the reaction. For example, a chiral catalyst is more likely to produce a stereoselective product than an achiral catalyst.
Methods for Predicting Stereochemical Outcome, Predict the stereochemical outcome of the following reaction
There are several methods that can be used to predict the stereochemical outcome of a reaction. These include:
- Molecular mechanics:Molecular mechanics is a method that uses computational techniques to calculate the energy of a molecule. This information can be used to predict the stereochemical outcome of a reaction.
- Quantum mechanics:Quantum mechanics is a method that uses quantum theory to calculate the energy of a molecule. This information can be used to predict the stereochemical outcome of a reaction.
- Experimental methods:Experimental methods, such as NMR spectroscopy and X-ray crystallography, can be used to determine the stereochemical outcome of a reaction.
Applications of Stereochemical Outcome Prediction
Stereochemical outcome prediction is used in a variety of fields, including:
- Organic chemistry:Stereochemical outcome prediction is used in organic chemistry to design and synthesize molecules with specific properties.
- Biochemistry:Stereochemical outcome prediction is used in biochemistry to understand the structure and function of biological molecules.
- Drug design:Stereochemical outcome prediction is used in drug design to design and synthesize drugs with specific biological activity.
Case Study: Predicting the Stereochemical Outcome of a Specific Reaction
Consider the following reaction:
Substrate:(R)-2-bromobutane Reagent:NaOH Product:(S)-2-butanol
Using the methods described above, we can predict the stereochemical outcome of this reaction. Molecular mechanics calculations show that the transition state leading to the (S)-2-butanol product is lower in energy than the transition state leading to the (R)-2-butanol product.
This suggests that the (S)-2-butanol product is more likely to be formed.
Experimental methods, such as NMR spectroscopy and X-ray crystallography, can be used to confirm the stereochemical outcome of this reaction.
FAQ Summary
What is the significance of stereochemical outcomes?
Stereochemical outcomes determine the physical and chemical properties of molecules, influencing their biological activity, reactivity, and stability.
How do factors such as temperature and solvent affect stereochemical outcomes?
Temperature and solvent can influence the reaction rates and equilibrium positions, thereby affecting the stereochemical outcome.
What are the advantages of using computational methods to predict stereochemical outcomes?
Computational methods can provide accurate predictions for complex reactions and can be used to screen potential reaction pathways.